Multielectrode Array (MEA)
✓ Measure electrical signals at multiple locations throughout the culture
✓ Mimic biophysical in vivo microenvironment
✓ Lowest cost multielectrode array electrophysiology data acquisition system
One of the substantial weaknesses of in vitro experiments is their failure to replicate the conditions of cells in an organism. Isolated and cultivated primary cells on rigid MultiElectrode Arrays (MEAs) differ significantly from the corresponding cell type in vivo, limiting the predictive value of in vitro data. BMSEED’s stretchable MultiElectrode Arrays (sMEAs), which contains elastically stretchable electrodes embedded in an elastomeric matrix that contact the cell or tissue culture, address this challenge by reproducing the mechanical and electrical environment of cells in vivo in a controlled environment in vitro. Stretchable multielectrode arrays enable mechanical stretching, optical imaging, and electrical stimulation/recording, bridging the gap between in vitro and in vivo research to enhance the relevance of experimental data.
Why Our MultiElectrode Arrays?
How stretchable multielectrode arrays improve physiological relevance of in vitro research
✓ Reduced cost and complexity compared to other electrophysiological techniques
✓ Real-time, in-situ, and label-free measurements
✓ Suitable for dissociated cell cultures, slices, and organoids
✓ Record from up to 120 microelectrodes simultaneously
✓ Chronic and acute experiments
✓ Mimic biophysical in vivo microenvironment to more accurately predict in vivo behavior using in vitro data
✓ Electrically stimulate cells in culture
✓ Largest variety of multielectrode arrays (2D, 3D, soft, traditional)
✓ Assess efficacy and toxicity of drugs
Our team of experts...
Founder
Phone: +1 (609) 532-9744
Email: oliver@bmseed.com
Application Fields of MultiElectrode Arrays
(I) Better Predict in vivo Behavior with in vitro Data
Biomimetic soft substrates (as opposed to glass or plastic)
Mimic bio-mechanical and bio-electrical microenvironment of cells in vivo
Use hiPSC-derived human cells
Tight control of experimental parameters for repeatable and reproducible experiments
BMSEED’s stretchable multielectrode arrays (sMEAs) are the ONLY multielectrode arrays that allow the application of mechanical cues in conjunction with electrophysiological measurements.
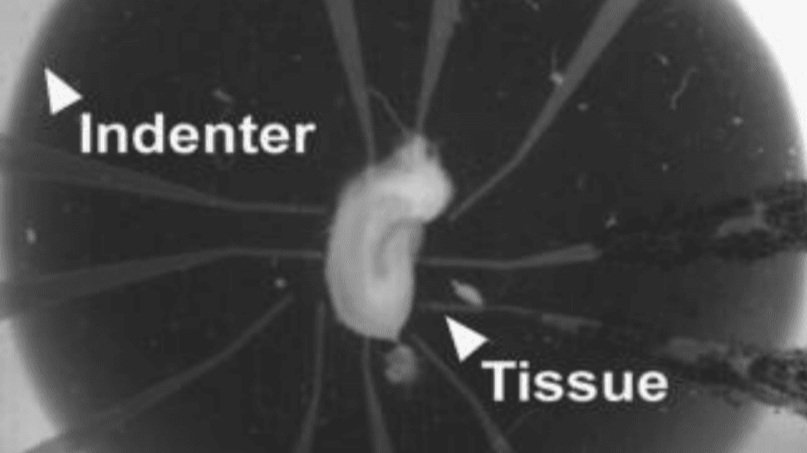
(II) Neurotrauma Research: Traumatic Brain Injury (TBI) & Spinal Cord Injury (SCI)
Mimic stretch and compression injury in vitro using dissociated cell or tissue slice cultures
Directly assess the impact of the injury on cellular health and function using non-invasive electrophysiology measurements
Directly compare post-injury electrophysiology with pre-injury data
Apply multiple injuries, e.g., to mimic repeated concussions
Apply different strain profiles (radial, uni-axial, other) to mimic different types of injury using the same equipment
Highly reproducible parameters for repeatable experiments
Assess neuroprotective properties of drugs to mitigate the damage after the injury
BMSEED’s stretchable multielectrode arrays (sMEAs) are the ONLY multielectrode arrays that are compatible with stretch injury models.
(III) Cardiac Research
Improve maturity of cardiac cells to exhibit an adult-like phenotype by applying bio-mechanical cues to the culture
In vitro results for efficacy and toxicity of drugs are more predictive of in vivo behavior (reduced failure rate for clinical trials)
Monitor cardiac differentiation and maturation in vitro using label-free electrophysiology measurements
Use electrical stimulation for controlled pacing of cardiomyocytes
Evaluate cardiotoxicity and proarrhythmic effects of drug candidates
BMSEED’s stretchable multielectrode arrays (sMEAs) are the ONLY multielectrode array technology that combines mechanical and electrical stimulation of cell cultures with electrophysiological measurements.
(IV) Other Application Fields of MultiElectrode Arrays
Mechanobiology
Bottom-up neuroscience
Pain research
other
Ask us whether your research would benefit from using multielectrode arrays.
What Clients Say About our MultiElectrode Arrays
“Tuneable parameters and very fast to use.”
-A. Pybus, Ph.D. Georgia Institute of Technology
“Easiness for acquiring electrical signal in a non-invasive manner.”
-A. Patino, Ph.D. Arizona State University
“The most valuable thing is that we can record from cells multiple times…injure and record on the same device.”
-M.K. Dwyer, M.S. Columbia University
a) organotypic hippocampal slice cultures (OHSCs), long-term potentiation (LTD) & long-term depression (LTD) induced post-injury; b) primary hippocampal neurons (PHNs) & brain tissue tolerance to traumatic brain injury (TBI); c) organotypic spinal cord slice cultures (OSCSCs) & cypin regulated pain sensitivity after spinal cord injury (SCI); d) human induced pluripotent stem cell-derived cardiomyocytes (hiPSC-CMs), hiPSC-CM differentiation; e) neurons, astrocytes, & microglia, pro-inflammatory signaling & immune response to mild traumatic brain injury (mTBI)
Our team of experts...
Founder
Phone: +1 (609) 532-9744
Email: oliver@bmseed.com
MultiElectrode Array Systems
BMSEED’s MEA Electrophysiology Data Acquisition System is available as a stand-alone equipment or integrated with a cell stretcher and imaging capabilities (MEASSuRE platform)
Stand-Alone
120 channel electrophysiology controller
60 or 120 channel recording and stimulation of electrophysiological activity
Stimulation currents from 10 nA - 2.55 mA
Analog and digital stimulation triggers
Compatible with stretchable multielectrode arrays (sMEAs) and glass multielectrode arrays (gMEAs)
Low-cost, high performance data acquisition system for electrophysiology
Contact us for pricing information.
Integrated in MEASSuRE Platform
Mechanics Module: stretching at strain rates up to 90/s and strains up to 50%
Imaging Module: optical or fluorescence imaging during stretching
Electrophysiology Module: 2x60 channels for recording and stimulation
Contact us for pricing information.
Frequently Asked Questions About our MultiElectrode Arrays
1. What is a multielectrode array?
Tools for Electrophysiological Recording and Stimulation
Multielectrode arrays (MEAs), also known as microelectrode arrays, are microfabricated platforms designed for electrophysiological investigations. They consist of a dense grid of small electrodes fabricated from biocompatible and electrically conductive materials, such as gold, platinum, or titanium. These electrodes are typically deposited onto a substrate material offering varying degrees of rigidity (glass), flexibility (polyimide), or stretchability (PDMS).
Multielectrode arrays offer a versatile tool for studying excitable tissues both in vitro (cultured cells) and in vivo (living organisms). Their primary function involves:
Electrophysiological recording: By virtue of their close proximity to living cells, multielectrode arrays passively capture the minute voltage fluctuations associated with cellular activity (e.g., action potentials, synaptic events) in the extracellular space.
Electrical stimulation: Multielectrode arrays can also be employed to deliver controlled electrical currents to stimulate cells, enabling researchers to probe cellular responses or modulate network activity.
The broad applicability of multielectrode arrays, including both traditional rigid and novel stretchable configurations offered by BMSEED, allows researchers to investigate diverse biological phenomena, ranging from cellular electrophysiology and network dynamics to drug discovery applications.
2. How does a multielectrode array work?
Multielectrode arrays are critical components in the process of acquiring electrical signals from cells. We describe briefly the process of how multielectrode arrays are used in recording and stimulation of electrophysiological activity.
Cellular Interface: The multielectrode array surface is typically composed of biocompatible materials and presents a microfabricated grid of microelectrodes (e.g., gold, platinum) for intimate contact with cultured cells. This biocompatibility enables acute and long-term (months) electrophysiological studies.
Extracellular Recording: As excitable cells (neurons, cardiomyocytes) adhere and form networks on the MEA, their electrical activity (action potentials, synaptic events) generates minute voltage fluctuations (typically 10s to 100s of μV) in the extracellular space.
Signal Transduction: These voltage changes are passively captured by the electrodes due to their close proximity to the cells. The microelectrodes on the multielectrode array basically transduce ionic currents in the medium that are caused by cellular electrical activity into electronic currents.
Signal Processing: The acquired signals are weak and require amplification to enhance their amplitude for further analysis. The amplifiers on the headstage amplify the differential signal of recording and reference electrodes. The amplifiers on BMSEED’s MEA electrophysiology system are placed in proximity to the electrodes to minimize noise.
Noise Elimination: Filtering techniques are employed to eliminate unwanted electrical noise originating from the environment or the multielectrode array itself. BMSEEED’s multielectrode array electrophysiology system has a notch filter to eliminate 50Hz and 60Hz line noise, as well as low-pass filters and high-pass filters with adjustable frequencies.
Data Acquisition: The amplified and filtered signals are then digitized and transferred to a computer system for further processing and analysis.
Electrophysiological Analysis: Specialized software allows researchers to analyze the recorded activity, including spike sorting (identifying individual neuron activity), network analysis (evaluating communication patterns), and field potential characterization (understanding overall network behavior). Ask BMSEED for the NeuroExplorer software.
Multielectrode arrays offer a minimally invasive approach for studying large ensembles of excitable cells, providing valuable insights into cellular electrophysiology, network dynamics, and drug discovery applications.
-
Multielectrode arrays (MEAs) offer several key benefits for electrophysiological measurements:
Network Analysis: MEAs record from multiple electrodes simultaneously, enabling researchers to gather data from a large number of cells or across a larger tissue area compared to single-electrode techniques. This capability allows researchers to analyze the communication patterns and synchronization within neural networks or other excitable cell cultures.
Label-Free: MEAs enable the recording of electrophysiological signals without the use of any labeling dyes (e.g., voltage sensitive dyes). These dyes, e.g., voltage-sensitive dyes, may affect how the cells function and thus the results of the experiment. Using MEAs avoids these issues.
In-Situ, Real Time: The presence of the multiple electrodes on an MEA does not affect the physical environment of the cells for in vitro analysis. The electrophysiological data are displayed in real time.
No Tissue Damage: In vitro studies using MEAs avoid the need for insertion of electrodes into the tissue, thus reducing potential damage to the tissue.
Extracellular Recording: MEAs capture the electrical activity of cells from the surrounding extracellular space, offering a non-invasive alternative to intracellular patch-clamp recording.
Drug Discovery: The ability to monitor cellular activity in response to drugs or compounds makes MEAs valuable tools for drug screening and development.
MEAs provide a powerful and versatile platform for studying cellular electrophysiology and network dynamics, offering significant advantages over traditional single-electrode techniques.
-
Multielectrode arrays record the electrical activity from the extracellular space of a population of cells (neurons, muscle cells) whereas patch clamp electrophysiology records the action potential electrical activity from the intracellular space of a singlecell.
Advantages
Recording data points from a network/population of cells simultaneously
Analysis of cellular network activity and synchronization
Coverage of a large area (several millimeter)
Non-invasive
Relatively easy to use
Relatively inexpensive
Disadvantages
Lower signal to noise ratio than patch-clamp.
-
BMSEED is the only company that offers stretchable multielectrode arrays (sMEAs) for in vitro research and drug development applications. Below we outline the advantages of using stretchable multielectrode arrays for in vitro studies.
Mimicking in vivo conditions: In vitro studies ultimately aim to predict in vivo behavior. There are many reasons for using in vitro studies instead of carrying out in vivo studies directly. Compared to in vivo studies, in vitro studies provide: (a) reduced cost, (b) increased speed, (c) tight control of chemical and physical environment, (d) higher throughput, (e) reduced animal-use, and (f) evaluation of biological phenomena without the potential confounding variables present in whole organisms. The drawback of in vitro studies is that the cellular environment is often very different from the environment in vivo that the cells experience. This difference causes cells to often behave differently in vivo than in vitro. BMSEED’s stretchable multielectrode arrays aim to reduce the difference between in vivo and in vitro cellular environments by replicating biophysical aspects of the living tissue environment in a controlled environment in vitro.
Improved cell viability: Rigid multielectrode arrays can exert pressure on cultured cells, potentially affecting their health and viability. The conformability of stretchable multielectrode arrays can minimize this stress, leading to healthier and more representative cell cultures for in vitro studies.
Studying dynamic cell cultures: Certain in vitro models involve culturing cells on flexible substrates that mimic specific tissues or organs. Stretchable multielectrode arrays can better integrate with these dynamic environments, maintaining good contact with the cells as they move or deform.